Silicate Mineral Structural Styles
 |
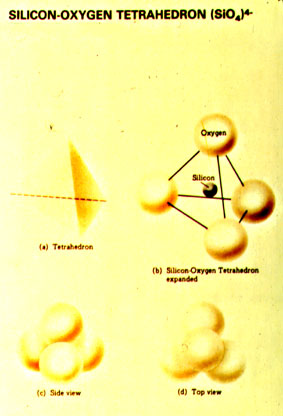
Under the pressure temperature conditions of the crust and mantle, silicon and oxygen will usually combine to form the complex ion SiO44-, which geometrically is a tetrahedron with silica in the center and oxygen at the four corners (a tetrahedron is a geometric shape having four triangular-shaped sides). Linking these tetrahedra on the corners, either by sharing adjacent oxygens, or by inserting positively charged metal ions between tetrahedra, allows a great many crystal structures to be constructed. |
Basically, silicate minerals show six different structural styles:
All, except the ring structures, are found in our rock forming minerals. Despite the limited number of components, the large numbers of resulting silicate minerals have very distinct crystalline structures, and equally distinct physical and chemical properties. Thus, with a very small number of components a vast array of different compounds and structures can be produced. In that sense the versatile silicone plays in the world of inorganic chemistry a similar role as carbon does in the world of organic chemistry. And indeed, the two elements are direct neighbors in the periodic system and thus show many parallels in their chemical behavior. It is for that reason that some people (scientists, and in particular science fiction writers) have speculated that carbon based lifeforms (like on our planet) may not be the only way to construct living organisms, and that there may be silicon based lifeforms out there, just waiting to be discovered.
WHERE DOES OUR KNOWLEDGE OF THE CRYSTAL STRUCTURES COME FROM?
Atoms are very small (on the order of 10-9 mm or 10-12 m), and we can not see them with a microscope. Visible light has a wave length around 400 to 700 10-9 m, about 1000 times larger. Because the theoretical optical resolution an imaging system is by physical laws limited to 1/2 the wavelength used for observation, visible light is in a way "too coarse" for the observation of atoms. We can get to smaller wavelengths when we use an electron microscope, but even very fast electrons have an insufficiently small wavelength. High energy X-rays have a wave length that is small enough to image atoms, but the problem is that they penetrate pretty much everything and are very difficult to manipulate optically. However, X-rays interact with the atoms of a crystal as they pass through it. In certain directions the X-rays will be blocked, whereas in other directions they can pass or are reflected by the crystal lattice. This method of observing crystals is called X-ray diffraction. If we place a photographic film around a mineral that has an X-ray beam shot at it, these directions of passage and reflection will produce a pattern of lines that will be arranged with characteristic distances for each mineral. From the line spacings it is possible to calculate the spacing and arrangement of crystal planes (lattice planes), and finally the spacing of the atoms in a mineral. Although invented early this century, X-ray diffraction is still a widely used method to study the internal structure of all sorts of solids. For example, the determination of the atomic structure of DNA molecules was accomplished by using X-ray diffraction (uses crystallized DNA). The scientists who figured out how to do this won the Noble Price in chemistry.
 |
The picture at the left is a X-ray diffractogram of quartz. The diffraction lines are not all of the same intensity, and the intensity patterns as well are characteristic (and unique) for each mineral. |
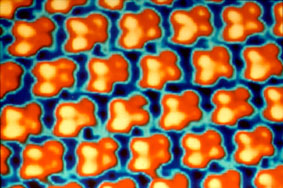 |
A more recently developed tool to image atoms is the Scanning Tunneling Microscope. This kind of microscope uses a very tiny probe (needle) that is moved across a sample surface and measures the electron distribution (intensity of the electric field, or more accurately the flow of "tunneling" electrons). Because atoms have electrons on the outside, they show up as roundish balls. The picture at left is an image of clusters of Sulfur atoms (4 atoms each), that are attached to a Rhenium surface. It does show the regular arrangement of the Sulfur clusters (due to the crystal structure of the Rhenium substrate), and the fourfold nature of the Sulfur clusters. Although the Scanning Tunneling Microscope allows us to image the geometric arrangement of atoms directly, it only allows us to image surfaces. In order to figure out the 3D arrangement of atoms in a mineral we still have to rely on X-ray diffraction. |