The Origin of Magmas
Magma forms by partial melting of upper mantle and crust. Partial melt means that only a fraction of the available material forms a melt, and that the remainder stays solid. The partial melt rises because of its lower density and ascends through he crust. How do partial melts form? Won't the source material just melt all together when the right temperature is reached? The reason why the mantle and crust can form partial melts is because they are not homogeneous substances. Instead, they consist of an assemblage of several substances (minerals). From daily life we know that a mixture of two or more substances has the effect of lowering the melting point (example: solder, a mixture of lead and tin, melts below the melting point of either lead or tin).

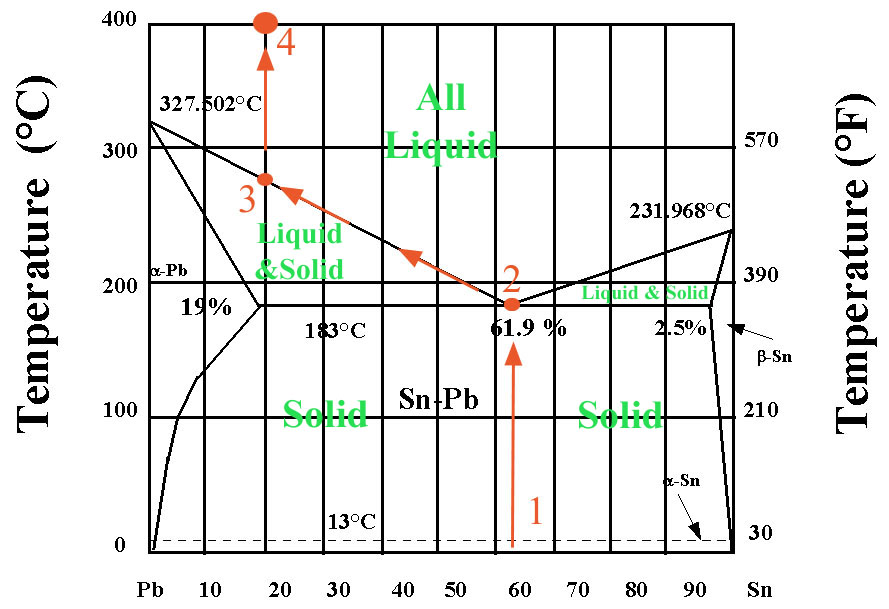
The Lead-Tin mixture in solder constitutes a binary system (meaning it has two components). Above diagram shows the temperature behavior of the system. In the lower portion of the temperature range (up to 183 degrees C) we have a solid Pb-Sn alloy. Above that Temperature we have a mixture of liquid and solid, except if we have a mixture with 61.9% Sn and 38.1% Pb. If that mixture is heated it turns into liquid directly (this by the way is the composition of high quality solder). If we assume to heat a mixture that contains 20% Sn and 80% Pb (composition at second vertical line from left edge of diagram), the first melt that forms will form when the lowest point of the melt diagram is reached (point 2). This point is also called the eutectic. The first melt is (Sn/Pb = 61.9/38.1)very different from the composition of the solid (Sn/Pb = 20/80) that it forms from. However, only a small portion of the solid has melted (thus partial melt). As we heat further, more and more of the Pb will melt, and the composition will creep to the left (towards point 3), as indicated by the red arrows. Finally, when we reach point 3, the last bit of lead has melted and we have a complete liquid (Sn/Pb = 20/80). With further heating no more compositional changes will occur, only the temperature of the mixture will rise. One of the inherent properties of such a system is this: no matter what the composition of the solid mixture is, the first melt will always be the same (Sn/Pb = 61.9/38.1).
How does this apply to rocks? Well, if we had for example a rock composed of a mixture of two minerals it would behave quite analogous to our Pb/Sn system. It gets more complicated of we have more than two minerals in the system (typical for rocks), and we have to imagine all this to happen in a multidimensional (and thus unimaginable) diagram. That does not stop us, however, from calculating what goes on in this multi phase space. Also, it is still true that the first melt will be of a composition that is fixed by the participating components, and not by the actual composition of the source rock. Widely different source materials, as long as they all contain the same mineral assemblage, will still give rise to the same partial melt.
What this means for magma generation is this: although the upper mantle
(continental crust, ocean crust) varies somewhat in composition, because it contains the
same minerals throughout, it will produce very similar partial melts worldwide.
Partial melting of mantle rocks will always produce magmas of basaltic composition.
Partial melting of subducted ocean crust (basalt) will always produce magmas
of andesitic composition. Partial melting of lower continental crust (on
average of andesitic composition) will always produce magmas of granitic composition.
Instead of generating a whole spectrum of magmas through melting of crust and mantle,
there are only three principal magma families, each with a limited compositional range.
FACTORS THAT INFLUENCE PARTIAL MELTING: