Atoms & Isotopes; Compounds & Minerals
 |
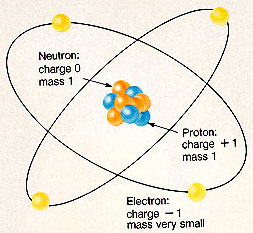
Atoms are the smallest fraction of an element that can exist, and still show the characteristics of the element. Atoms themselves are composed essentially of electrons (1 negative charge), protons (1 positive charge), and neutrons (no charge). In a simplistic way we can visualize atoms as consisting of a nucleus with protons and neutrons that is surrounded by electrons for charge balance. Atoms of different elements are distinguished by the number of protons in the nucleus. Hydrogen has the simplest (and lightest) atom with just one proton and one electron, and then complexity gradually increases until we get to Uranium with 92 protons, 146 neutrons, and 92 electrons. |
Normally the number of electrons equals the number of protons, however an atom may loose or pick up electrons and have a negative or positive charge, in which case we talk about ions rather than atoms.
Although the "planetary" model of the atom as shown above is easy to visualize, reality is a bit stranger. According to a quantum mechanic approach pioneered by Schrödinger, the behavior of electrons can be envisioned as probability functions of wave forms. If the probabilities are plotted in 3D, they look like the animated pictures above. All of the pictures describe electron distribution of a hydrogen atom in various energy states (quantum states, states if "excitation). |
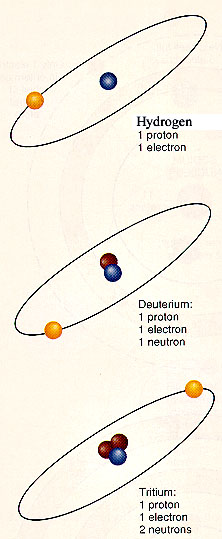
The number of neutrons (the "glue" between the protons) in the nucleus may
also vary within a given element. These varieties of a given atom (same number of protons,
different number of neutrons) are called isotopes. The illustration
at the left shows three isotopes of hydrogen: "normal: hydrogen with 1 proton and 1
electron, deuterium, and tritium. Scientists in various laboratories are working to
produce controlled fusion of deuterium into helium. If successful, this will
be an almost inexhaustable source of energy. Although deuterium constitutes only a small
fraction of the hydrogen on Earth (a few liters in every cubic kilometer of ocean water),
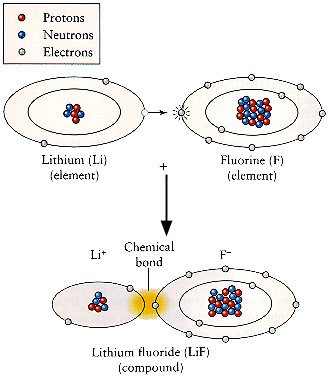
there is a large volume of ocean water to draw on. Atoms can join together to form molecules. Most of the substances around us consist of molecules and are called compounds of various elements. Molecules are the smallest particle of a compound that has all the chemical properties of that compound. They are made up of two or more atoms, either of the same element or of two or more different elements. Molecules and compounds contain their constituent elements in specific proportions that are characteristic of the compound. For example, water (H2O), a well known and simple compound, contains hydrogen and oxygen at a ratio of 2 to 1. The illustration below shows how Lithium and Fluorine combine to form the compound Lithiumfluoride. In essence, Lithium gives up an electron and becomes positively charged, whereas Fluorine picks up this electron and becomes negatively charged. The attraction between the oppositely charged ions causes them to join in a so called ionic bond. |
|
 |
Other types of bonds are the covalent bond (atoms share electrons) and the metallic bond (atoms are surrounded by an "electron soup"). Which bond is found in a given compound depends on the elements that are present and the chemical characteristics of the elements involved.
The materials that make up the Earth crust and mantle are called rocks (there is a great variety of them), and these rocks are composed of a mixture of pure elements (e.g. diamonds [pure carbon], gold) and various chemical compounds of silica, oxygen, iron, magnesium, aluminum, etc. As said above, each chemical compound shows very specific proportions of elements that it is composed of. These proportions depend on the electron configuration of the participant elements. One particular thing about the compounds that compose rocks is that they not only show compound-specific proportions of elements, but also that each has a compound specific internal arrangement of atoms. Because of this regular arrangement of atoms they are crystalline substances. We call this type of compounds minerals. Minerals are the main building blocks of all rocks.
By definition, minerals have the following characteristics:
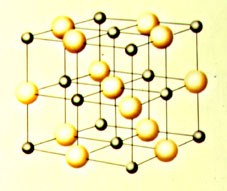 |
The crystal structure of cooking salt, Sodium Chloride (NaCl). The structure consists of alternating Sodium ions (positive charge, small black balls), and Chlorine ions (negative charge, large yellow balls). In this case the ions are laid out on rectangular grids, in three dimensions we have a cubic structure. Depending on the relative size of the ions we get different structural angles and structural types. |
By above definition, all minerals have a crystalline structure. Not all crystalline substances, however, are necessarily minerals. Sugar, for example, forms very nice crystals, but it is not a mineral because it is an organic substance. Minerals are inorganic substances.
Minerals have specific physical properties that are used to distinguish and classify them. These properties are:
These properties are a function of the bond strength, the internal structure, and the chemical composition of the mineral.
 |
Cleavage in Calcite (CaCO3). No matter how often the calcite crystal is broken into smaller and smaller pieces, the resulting fragments always show rhombohedral cleavage. This is so because of the internal arrangement of atoms in the calcite crystal. Cleavage angles are a fundamental property of any given mineral. |
 |
A given compound may occur in more than one crystal structure. The picture on the left shows a diamond and graphite (used in pencils). Both consist of pure carbon (C), but differ markedly in their properties. Graphite is soft, shiny dark gray, and has a layered, sheetlike, internal structure. In contrast, diamond is denser, often transparent, and the hardest substance known to man. The difference lies in the 3D framework structure of diamond that gives it lots of strength and structural integrity (the reason is that diamond forms under the very high pressures of the upper mantle). When a compound can form more than one type of crystal structure it is called polymorphous. Diamond and graphite are polymorphs of carbon. |